In this area we concentrate on developing Therapeutic Ultrasound (TUS) as an efficient alternative to viral and chemical delivery of nucleic acids. We were the first to show that long-term application of TUS can drive cDNA into cell nuclei, and demonstrated the durability of acoustic contrast agents (Optison™), shown to enhance transfection in a non-cavitational mechanism using such conditions. We were also the first to apply TUS-mediated delivery of cDNA encoding for angiogenic inhibitors (PEX) leading to substantial inhibition of prostate cancer progression. Additional efforts are focused on developing nano gene delivery system either targeted or for sustained local release of therapeutic genes.
Biodistribution of TUS transfected-MSC
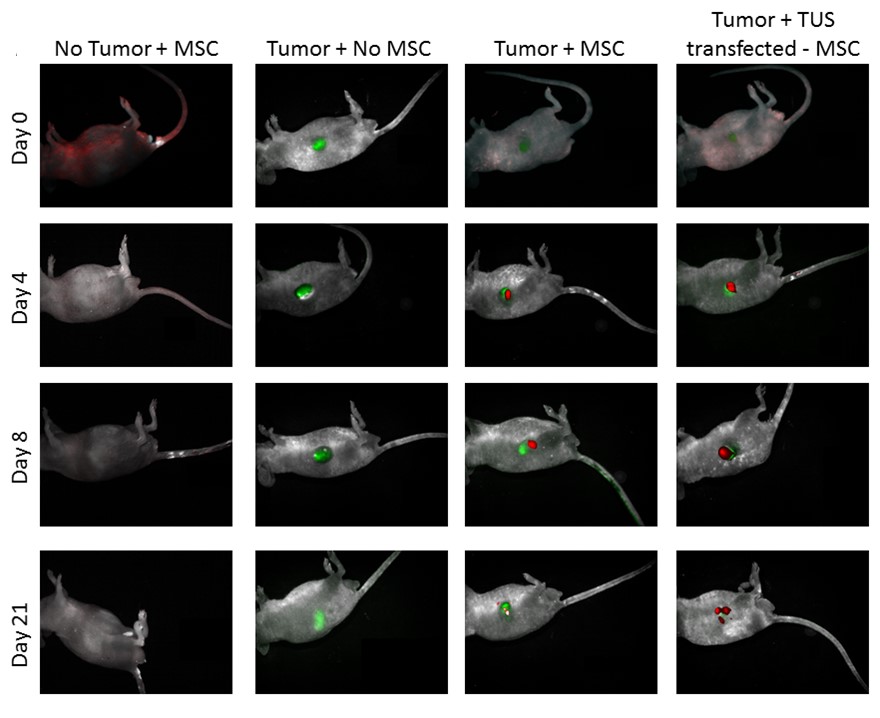
TUS-PEX-transfected MSC (green) administered to tumor (red) bearing mice. While immediately after the administration (day 0), the MSCs can be found all over the body, four days post administration MSCs can be found mainly around the tumor. The accumulation of MSCs around the tumor becomes clearer 8 days post administration, in which the MSC can be found only around the tumor site and with no traces in other organs. The MSC can be found around the tumor site even after 21 days.
SELECTED PUBLICATIONS
Mesenchymal stem cells (MSCs) hold tremendous potential as a targeted cell-based delivery platform for inflammatory and cancer therapy. Genetic manipulation of MSCs, however, is challenging, and therefore, most studies using MSCs as therapeutic cell carriers have utilized viral vectors to transduce the cells. Here, we demonstrate, for the first time, an alternative approach for the efficient transfection of MSCs; therapeutic ultrasound (TUS). Using TUS with low intensities and moderate frequencies, MSCs were transfected with a pDNA encoding for PEX, a protein that inhibits tumor angiogenesis, and studied as a cell vehicle for in vivo tumor therapy. TUS application did not alter the MSCs’ stemness or their homing capabilities, and the transfected MSCs transcribed biologically active PEX. Additionally, in a mouse model, 70% inhibition of prostate tumor growth was achieved following a single I.V. administration of MSCs that were TUS-transfected with pPEX. Further, the repeated I.V. administration of TUS-pPEX transfected-MSCs enhanced tumor inhibition up to 84%. Altogether, these results provide a proof of concept that TUS-transfected MSCs can be effectively used as a cell-based delivery approach for the prospective treatment of cancer.
Nonviral gene delivery methods encounter major barriers in plasmid DNA (pDNA) trafficking toward the nucleus. The present study aims to understand the role and contribution of therapeutic ultrasound (TUS), if any, in pDNA trafficking in primary cells such as fibroblasts and cell lines (e.g., baby hamster kidney [BHK]) during the transfection process. Using compounds that alter the endocytic pathways and the cytoskeletal network, we show that after TUS application, pDNA trafficking in the cytoplasm is not mediated by endocytosis or by the cytoskeletal network. Transfection studies and confocal analyses showed that the actin fibers impeded TUS-mediated transfection in BHK cells, but not in fibroblasts. Flow cytometric analyses indicated that pDNA uptake by cells occurs primarily when the pDNA is added before and not after TUS application. Taken together, these results suggest that TUS by itself operates as a mechanical force driving the pDNA through the cell membrane, traversing the cytoplasmic network and into the nucleus.
Background: One of the major limitations of nonviral gene delivery methods is nuclear transport of plasmid DNA (pDNA). Peptides bearing nuclear localization signal (NLS) were shown to mediate nuclear import of macromolecules. We have explored the use of cell-permeable peptides (CPP) bearing NLS sequences to enhance transfection mediated by a nonviral approach: therapeutic ultrasound (TUS). Methods: Two CPP-NLS peptides which differ in the location of the NLS relative to the CPP were used: S413-PV and PV-S413. The peptides were attached to pDNA using electrostatic interactions. Gel-electrophoresis and fluorescent assays were performed to evaluate pDNA–peptide interactions and condensation effects. Confocal microscopy was used to evaluate pDNA–peptide interaction inside cells. Transfection studies were conducted with the luciferase gene, using pDNA-peptides alone, or with the application of TUS. Results: Attachment of both peptides to pDNA condensed the pDNA, with higher affinity for the S413-PV peptide. This interaction protected pDNA from endonucleases, but was also reversible. Both peptides mediated pDNA delivery to cell cytoplasm, but less significantly to the nucleus. Thus, both peptides produced transfection in cells, when added after incubation with DNA, with higher transfection-level for PV-S413. Application of TUS increased transfection mediated by these peptides, but was not higher compared to transfection using TUS and pDNA alone. Conclusions: This study suggests that CPP-NLS peptides may be used for condensing pDNA and bringing it into the cell cytoplasm, but their ability to mediate nuclear import of pDNA is insignificant.
Gene therapy clinical trials are limited due to several hurdles concerning the type of vector used, particularly, the viral vectors, and transfection efficacy when non–viral vectors are used. Therapeutic ultrasound is a promising non–viral technology that can be used in the clinical setting. Here, for the first time, we show the efficacy of therapeutic ultrasound to deliver genes encoding for hemopexin-like domain fragment (PEX), an inhibitor of angiogenesis, to prostate tumors in vivo. Moreover, the addition of an ultrasound contrast agent (Optison) to the transfection process was evaluated. Prostate cancer cells and endothelial cells (EC) were transfected in vitro with cDNA-PEX using therapeutic ultrasound alone (TUS + pPEX) or with Optison (TUS + pPEX + Optison). The biological activity of the expressed PEX was assessed using proliferation, migration, and apoptosis assays done on EC and prostate cancer cells. TUS + pPEX + Optison led to the inhibition of EC and prostate cancer cell proliferation (<65%), migration (<50%), and an increase in apoptosis.In vivo, C57/black mice were inoculated s.c. with prostate cancer cells. The tumors were treated with TUS + pPEX and TUS + pPEX + Optison either once or repeatedly. Tumor growth was evaluated, after which histology and immunohistochemistry analyses were done. A single treatment of TUS + pPEX led to a 35% inhibition in tumor growth. Using TUS + PEX + Optison led to an inhibition of 50%. Repeated treatments of TUS + pPEX + Optison were found to significantly (P < 0.001) inhibit prostate tumor growth by 80%, along with the angiogenic indices, with no toxicity to the surrounding tissues. These results depict the efficacy of therapeutic ultrasound as a non–viral technology to efficiently deliver genes to tumors in general, and to deliver angiogenic inhibitors to prostate cancer in particular.
When designing a nonviral gene delivery system based on polymeric nanoparticles (NPs), it is important to keep in mind obstacles associated with future clinical applications. Simplifying the procedure of NPs production and taking toxicity into account are the most important issues that need to be addressed. Toxicity concerns in clinical trials may be raised when using additives such as cationic polymers/lipids, buffering reagents, and proteins. Therefore, the aim of this study was to simplify the formulation of poly (lactide-co-glycolide) acid NPs by shortening steps such as sonication time and by avoiding the use of additives while preserving its efficiency. NPs (300 nm) were formulated using a modified w/o/w technique with DNA entrapment efficiency of 80%. Once achieving such NPs, formulation parameters such as DNA loading, release kinetics, DNA integrity and bioactivity, uptake by cells, and toxicity were addressed. The NPs were readily taken by several cell lines and were localized mostly in their endo-lysosomal compartments. The NPs did not affect cells viability. Most importantly, transfection studies in COS-7 and Cf2th cells resulted with a 250-fold protein expression levels when compared with the control. These expression levels are higher than ones achieved with more complicated NPs systems, demonstrating the efficiency of our simplified NPs for gene delivery.
The application of therapeutic ultrasound (TUS) in combination with contrast agents (USCA) to mediate gene delivery relies on the understanding of the bioeffects involved. The objective of this study was to evaluate the various bioeffects generated by albumin-coated microbubbles: Optison, an USCA, when applied with TUS operated for 10–30 min, on cells and on DNA transfection. This study reveals that Optison microbubbles were still acoustically active after long-term TUS application of 30 min. Optison enhances TUS-gene transfection by increasing the number of plasmids in the cells and also by distributing the plasmids to more cells, without significant decrease in cell viability. Optison also interacts with the DNA to further enhance transfection in a mechanism not necessarily involving cavitation. However, Optison affects mainly the cell cytoplasmatic membrane, without interfering with DNA intracellular trafficking. Using high-resolution scanning electron microscopy (HRSEM), the bioeffects on cell membrane induced by TUS–Optison were observed, demonstrating that Optison lead to a rougher surface, characterized by depressions that are reversible within 24-h post TUS. These effects are different from those observed when only TUS was applied. The findings from this study suggest that albumin-coated microbubbles enhances transfection when using TUS for 10–30 min, and that microbubbles play a major role in elevating cell transfection level and efficiency.
