We develop a variety of vehicles and controlled-release systems to address the major obstacles in delivering therapeutics to treat cancer. We were the first to reformulate PLGA particles to entrap unstable anti-angiogenic biologic (PEX) and synthetic (Gleevec) drugs, which were shown to reduce side effects and substantially improve the drugs’ efficacy in treating brain tumors. We have formulated PLGA nano-particles for DNA delivery as well as PLGA particles for cancer immunotherapy. We have shown that BMP-2 embedded in microspheres enhanced the osteogenic differentiation of adipose mesenchymal stem cells for bone regeneration. Additionally, we have shown that PLGA microspheres loaded with Cediranib (AZD2171) can be used to treat glioblastoma. We also focused on designing PLGA microspheres incorporated with a pore-forming agent and an antigen peptide as a unique cancer protein vaccination delivery platform.
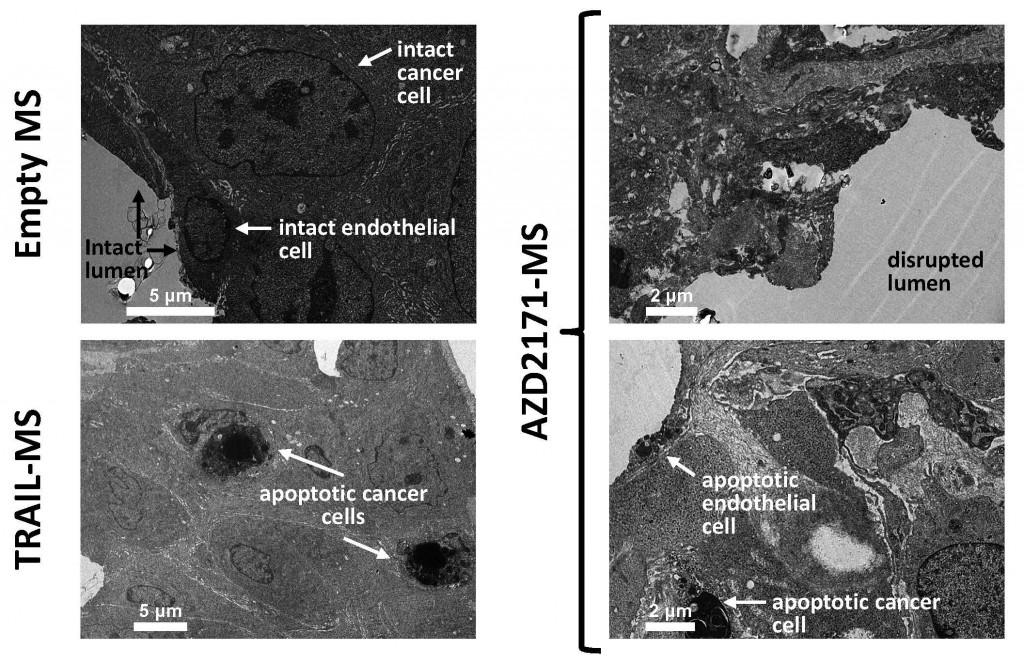
TEM micrographs of tumors explanted from mice treated with PLGA microspheres (MS) loaded with Cediranib (AZD2171) or aTRAIL/Apo2L
Cancer cells from mice treated with TRAIL-MS (bottom left panel) appeared to be more apoptotic than those from control mice injected with empty MS (upper left panel). Compared to the control animals (upper left panel), AZD2171-MS led to a more dramatic effect than TRAIL-MS, resulting in vast disruption of the blood vessels’ lumen (upper right panel) and more apoptotic cancer and endothelial cells (bottom right panel).
SELECTED PUBLICATIONS:
Strategies for cancer protein vaccination largely aim to activate the cellular arm of the immune system against cancer cells. Our approach aimed to examine the ability of listeriolysin O (LLO) incorporated into poly-lactic-co-glycolic acid (PLGA) microspheres to modify the cytosolic delivery of low molecular weight peptides and enhance their cross-presentation. PLGA microspheres were produced in a size suitable for uptake by phagocytic cells. The peptide encapsulation and release kinetics were improved by adding NaCl to the preparation. PLGA microspheres loaded with the antigenic peptide and incorporated with LLO were readily up-taken by phagocytic cells, which exhibited an increase in the expression of peptide-MHC-CI complexes on the cell surface. Furthermore, this system enhanced the activation of a specific T hybridoma cell line, thus simulating cytotoxic T cells. These results establish, for the first time, a proof of concept for the use of PLGA microspheres incorporated with a pore-forming agent and the antigen peptide of choice as a unique cancer protein vaccination delivery platform.
Studies with AZD2171-a new anti-angiogenic inhibitor of tyrosine kinases associated with VEGF signaling-have shown great promise for treating glioblastoma. Unfortunately, AZD2171 success is limited by low permeability through the blood-brain barrier. Due to AZD2171‘s short half-life and high toxicity, its local administration will require multiple intracranial procedures, making this approach clinically unfeasible. In this study, we investigated the potential of the highly hydrophobic AZD2171, released from modified polylactic-co-glycolic acid microspheres (PLGA-MS), to treat glioblastoma. To further demonstrate the versatile loading capacity of this system, the same PLGA formulation, which was found optimal for the loading and release of AZD2171, was tested with sTRAIL/Apo2L-a biologic drug that is very different than AZD2171 in its molecular weight, solubility, and charge.AZD2171 released from PLGA-MS was at least effective as the free drug in inhibiting endothelial growth and proliferation (in vitro), and, surprisingly, had a profound cytotoxic effect also towards in vitro cultured glioblastoma cell-lines (U87 and A172). Complete tumor inhibition was achieved following a single treatment with AZD2171-loaded PLGA-MS (6 (mg)/kg) administered locally adjacent to human U87 glioma tumors inoculated subcutaneously in nude mice. This improved effect, compared to other therapeutic approaches involving AZD2171, was shown to affect both tumor vasculature and the glioma cells. sTRAIL-loaded microspheres, administered at very low doses (0.3 (mg)/kg), led to 35 % inhibition of tumor growth in 2 weeks. Collectively, our results provide pre-clinical evidence for the potential of PLGA formulations of AZD2171 and sTRAIL to serve as an effective treatment for glioblastoma.
Purpose: In an effort to develop new therapeutic strategies to treat malignant gliomas, we have designed poly (lactic-co-glycolic) acid (PLGA) microparticles that deliver imatinib mesylate, a small molecule tyrosine kinase inhibitor. The local continuous release of imatinib mesylate at the tumor site overcomes many obstacles associated with systemic delivery. Experimental Design: Polymeric microspheres were prepared from various compositions of PLGA and loaded with imatinib mesylate. Imatinib release profiles, biological activity, and effect on PDGFR-B phosphorylation were confirmed in vitro. The therapeutic efficacy of imatinib microspheres was examined in two s.c. and orthotopic human glioblastoma xenograft models. Results: A single local injection of PLGA microspheres loaded with a low concentration of imatinib mesylate led to 88% and 79% reduction in s.c. human (U87-MG) and murine (GL261) glioma tumors, respectively. PLGA-imatinib mesylate administered intracranially led to a 79% reduction in U87MG tumor volume. Immunohistochemical analysis showed a marked decrease in proliferation indices and tumor vessel density in the s.c. model and induction of apoptosis in an intracranial model. Conclusion: This is the first study to show the therapeutic efficacy of the local delivery of imatinib mesylate using a polymeric delivery system.
The correlation between glioma grade and angiogenesis suggests that antiangiogenic therapies are potentially therapeutically effective for these tumors. However, to achieve tumor suppression, antiangiogenic therapies need to be administered daily using high systemic quantities. We designed a biodegradable polymeric device that overcomes those barriers by providing sustained local delivery of a C-terminal fragment of platelet factor 4 (PF-4/CTF), an antiangiogenic agent. Fluorescent-labeled microspheres composed of poly lactic-coglycolic acid (PLGA) were loaded with rhodamine-labeled PF-4/CTF and formulated to release their contents over time. Fluorescent labeling enabled the correlation between the in vitro to the in vivokinetic and release studies. PF-4/CTF microspheres were injected into established intracranial human glioma tumors in nude mice. Noninvasive magnetic resonance imaging (MRI) was used to assess the therapeutic response. Tumor size, microvessel density, proliferation, and apoptosis rate were measured by histological analysis. Intracranially, the microspheres were located throughout the tumor bed and continuously released PF-4/CTFduring the entire experimental period. MRI and histological studies showed that a single injection of microspheres containing PF-4/CTF caused a 65.2% and 72% reduction in tumor volume, respectively, with a significant decrease in angiogenesis and an increase in apoptosis. Our data demonstrate that polymeric microspheres are an effective therapeutic approach for delivering antiangiogenic agents that result in the inhibition of glioma tumor growth
Purpose: There is an urgent need for modalities that can localize and prolong the administration of the antitumor agents, particularly antiangiogenic, to achieve long-term tumor inhibition. However, one of the major obstacles is designing a device in which the biological activity of sensitive endogenous inhibitors is retained. We have designed a biodegradable polymeric device, which provides a unique and practical means of localizing and continuously delivering hemopexin (PEX) or platelet factor 4 fragment (PF-4/CTF) at the tumor site while maintaining their biological activity. The potential and efficacy of this system is shown in vitro and in vivo in a human glioma mouse model. Experimental Design: Polymeric microspheres made of poly(lactic-co-glycolic acid) (PLGA) were loaded with very low amounts of PEX and PF-4/CTF. The release profiles of these factors from PLGA and their biological activity were confirmed in vitro using proliferation assays done on endothelial and tumor cells. Tumor inhibition using this system was studied in nude mice bearing a human s.c. glioma. Results: PEX and PF-4/CTF released in vitro from PLGA microspheres were biologically active and significantly inhibited the proliferation of human umbilical vein endothelial cells, bovine capillary endothelial cells, and U87-MG cells. A single local s.c. injection of PLGA microspheres loaded with low amounts of PEX or PF-4/CTF resulted in an 88% and 95% reduction in glioma tumor volume 30 days post-treatment. Immunohistochemical analysis of the treated tumors showed a marked decrease in tumor vessel density compared with untreated tumors. Conclusion: Our findings show that polymeric microspheres are a very promising approach to locally and efficiently deliver endogenous inhibitors to the tumor site leading to a significant inhibition of the tumor.